12 questions to ask before purchasing a media preparator.
The culture media preparator is a professional equipment specially designed for producing large quantities of culture medium in microbiology laboratories and plant tissue culture laboratories. And its function is, as its name already indicates, to prepare the culture medium in which samples will be grown for microbiological analysis in the case of microbiology laboratories or explants in the case of companies dedicated to the cultivation of plant tissues.
When considering purchasing a media preparator for your laboratory, it is crucial to assess its capabilities and to determine the level of after-sales service provided by the manufacturer, both prior to and following the acquisition.
In the following article we will explain the key aspects that you should take into account when purchasing a media preparer for your laboratory. As you can imagine, at RAYPA we want to convince you of the advantages of our AE-MP Series media preparer because in addition to believing in its quality, efficiency and profitability , it is the culture media preparer that we know best. Shall we start?
Why should I purchase a media preparator?

Buying a media preparer is a large investment. And although in most cases the investment is recovered in a short time, the truth is that it means a significant economic effort. That is why before purchasing it, it is important to ask yourself the following questions:
1. Why does my laboratory need a culture media preparator?
It is possible that in your laboratory you produce culture media in large quantities manually, using an autoclave, and you do this process multiple times a week. If you are familiar with the process of preparing culture media, you are aware of its demanding nature in terms of labor, time consumption, staffing needs, and inherent risks. With over 15 years of experience in the field, we at RAYPA understand the challenges associated with manual media preparation. Many laboratories, similar to yours, have sought our guidance in automating this process to improve efficiency, reduce time consumption, and minimize the risks involved. Our extensive knowledge and expertise in media preparators have allowed us to provide insightful advice to numerous laboratories. As a consequence, you will experience significant time and cost savings, enhance the quality of your processes, reduce the possibility of human error, and minimize the risks of workplace accidents.
For this reason, we believe that we must be very clear and concise. RAYPA’s AE-MP Series media preparators allow you to carry out, in a single equipment, the 4 main phases of culture media production (preparation, sterilization, cooling and dispensing). As a result, the operational workflow will be optimized, the risk of contamination will be reduced, the total process time will be shortened, and large volumes of sterile culture media will be obtained, thanks to its efficient heating system and rapid cooling phase at the end of the sterilization process.
2. What types of culture media can I prepare?

The media preparator allows the production of all sorts of liquid and solid culture media, and some of the most common applications include Agar, buffer solutions and lysogeny broth.
With our media preparator you can prepare any type of culture media with any composition. In addition, it can prepare recipes with thermolabile nutrients or antibiotics thanks to the dispensing port installed on the cover, allowing the dispensing of these components after finishing the sterilization phase, avoiding the exposure of these compounds to temperatures higher than 70ºC. Additionally, the ability to program different temperature segments during the preparation process is a noteworthy feature, as it enables the preparation of more complex media recipes, such as Chocolate Agar.
Among the principal applications utilized by our customers, we observe the preparation of culture media for plant tissue culture, the preparation of Agar for qualitative microbiological analysis, and the preparation of lysogeny broth and buffer solutions for quantitative microbiological analysis.
3. What is the achievable productivity? How long does a cycle take?
As you can imagine, it is difficult for us to give you a concrete answer to this question because the duration of a cycle and the level of productivity will depend on the model of media preparator in question. As a rough guide, we can tell you that a complete cycle can last anywhere between 70 and 125 minutes. If you are concerned about this aspect, we have overpowered models with up a heating capacity of up to 30kW of electrical power that significantly shorten the duration of the heating phase, so shorter cycles can be performed.
In terms of productivity, i.e. the liters of culture medium that can be prepared per cycle, it can range from 5 to 90L, depending on the model of media preparator chosen.
Media Coach Model | Culture media preparation capacity* min. – max. L |
|---|---|
AE-20-MP-10L | 2 – 8 |
AE-20-MP | 2 -18 |
AE-40-MP | 5 – 36 |
AE-60-MP | 10 – 54 |
AE-80-MP | 20 – 72 |
AE-100-MP | 20 – 90 |
On the other hand, another key aspect is the dispensing speed at the end of a cycle, which in the case of the our media preparator ranges from 7ml/s to 100ml/s, depending on the chosen dispensing mode.
4. What size media preparer does my lab need?
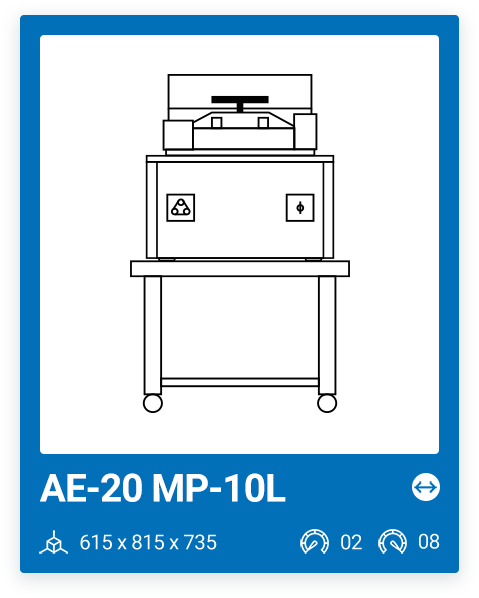

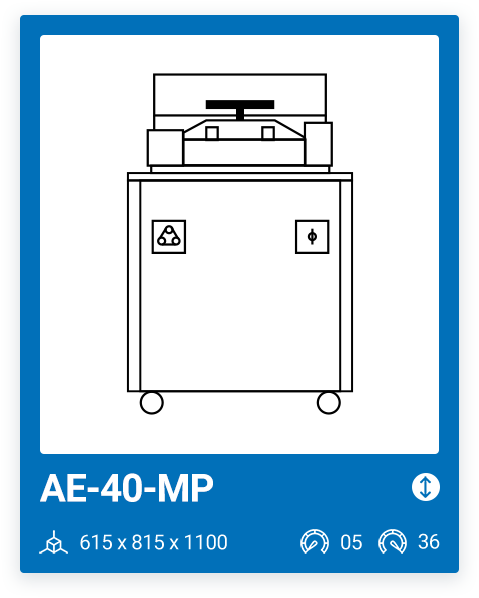
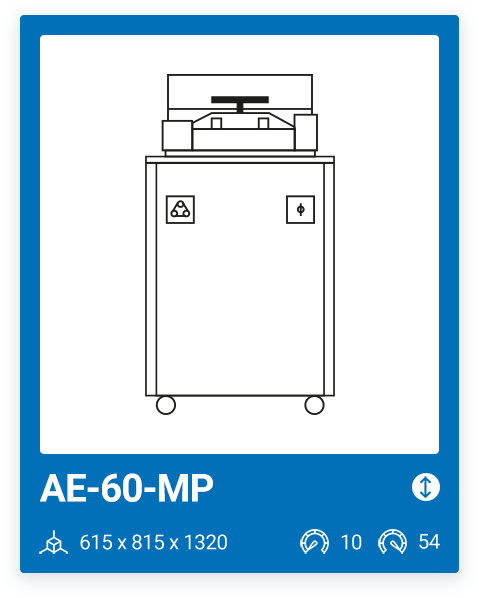
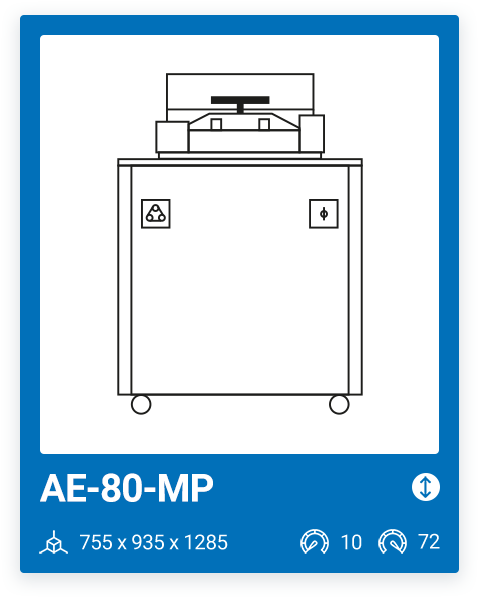
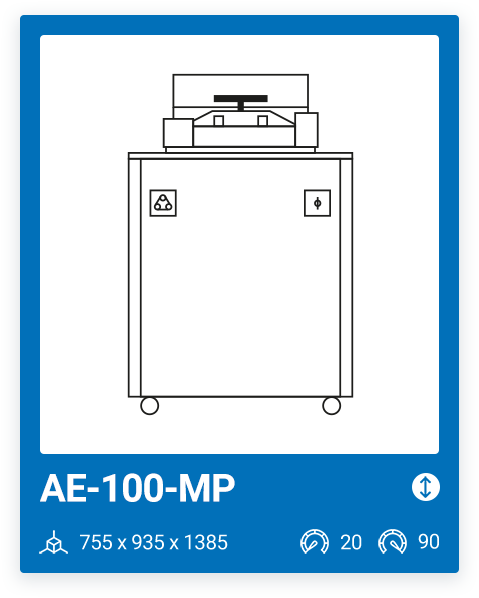



We offer a range of 6 models with varying capacities, ranging from a minimum capacity of 10 liters to a maximum chamber size of 100 liters. The most important factor when purchasing a media preparator and deciding on one model or another is your production needs. It is important that, in addition to your current needs, you also consider your future needs.
On the other hand, there are 3 other aspects to consider before choosing a model and configuration: electrical and water connection requirements, available space and the consumption of resources such as electricity and water.
5. What options are available for dispensing the culture medium?
To choose the appropriate dispensing mode you must first know what volume of medium you need to dispense per container. In the case of petri dishes, the laboratory operator can dispense the prepared and sterilized medium through an integrated peristaltic pump that is controlled by means of a foot pedal. Also, the execution of each filling can be programmed with a time delay.
In addition to this integrated peristaltic pump, all our models include a set of 2 dispensing lines of different diameters out of a total range of 5 (Ø3.2 4, 4.8, 6.4 and 8 mm). And, if you wish, you can add a second peristaltic pump to your unit in order to double the flow rate. Depending on the diameter of the chosen dispensing lines and the number of peristaltic pumps your media preparator has, the dispensing speed will range from 7 ml/s to 33 ml/s.
Additionally, for those laboratories that require the production of large quantities of culture media, our media preparators can equipped with pressure support and an external dosing station, enabling dispensing speeds ranging from 65ml/s to 100ml/s. When using this dispensing mode, the laboratory operator has the option to control the process through an optical sensor or a foot pedal.
And finally, it is important to note that dispensing can be paused and restarted if necessary without compromising the integrity of the process. The dispensing lines are emptied by counterpressure, avoiding clogging of the tube due to gelation of the culture medium. All of this, without affecting the temperature of the culture medium, still inside the preparer, because it is maintained indefinitely at the established degrees Celsius.
6. What is continuous steam used for?
The continuous steam cleaning system is a key differentiating factor of our media preparer with the rest of the equipment on the market. Thanks to this innovative system, the dispensing lines can be automatically cleaned and disinfected with continuous steam at 100ºC. Cleaning the lines can be done before, during or after the dispensing phase. This feature greatly facilitates cleaning the inside of the lines.
To carry out cleaning, you just have to press the corresponding button on the screen and keep the tip of the dosing tube inside a laminar flow cabinet, pointing it inside a container to avoid burns for the operators and treat the tip of the tube with a Bunsen burner. .
In fact, many of our clients have confessed to us that after disinfecting the lines in this way and working inside the cabin, they do not usually sterilize the dispensing lines in an autoclave. What they always do, both before starting to dispense and when they finish, is passing continuous steam through the entire line.
7. Is it possible to obtain lot-by-lot traceability?

With our media preparator you will be able to measure and control the different stages of the media production process in a simple and automatic way thanks to a specific software. Additionally, you will have the capability to document each batch independently and possess user administrative control. Furthermore, an embedded printer can be incorporated into the control panel to print tickets of each batch.
8. What connections are required to install the media preparator?
To install our media preparator in your laboratory you will only need:
Please note that we offer multiple heating capacities ranging from 3kW in the smaller models to 30kW in the 80 and 100 liter models. These units with improved heating capacity are especially useful for plant tissue laboratories that prepare large volumes of culture media.
A decalcified water network should be connected directly to the cooling water inlet that feeds the water cooling coils.
Regarding the water used for sterilization, it should be noted that you can either add the purified water manually by pouring it directly into the sterilization chamber or you can automate this task by connecting the equipment to a pressurized purified water network.
If you choose the second option, water should have a minimum pressure of >3.5 bar, a hardness of ≤0.02 mmol/l and a conductivity of 5< X≤15 μS/cm. Therefore, according to the type of installation, 1 or 2 water inlets will be required.
Un desagüe para el drenaje de la cámara de esterilización y también del agua de la refrigeración.
9. What is the technical support and warranty like? Who offers it?
If there is one thing that sets us apart from the rest, in addition to the performance of our equipment, it is the technical support we offer to our customers, both before and after the acquisition of a media preparator. For RAYPA, it is of utmost importance that its personnel is actively engaged throughout the entire process of each media preparator, including the pre-sale stage, manufacture, installation, and post-sale. In addition, we also grant access and involve our team of engineers to better serve our customers. Such involvement is so important because we know that, although the use of our media preparators is quite simple and intuitive, doubts may arise regarding their installation, software and/or maintenance.
Additionally, our technical service is always available to assist with any questions or technical issues, whether through a video call or on-site as necessary.
Additionally, after purchasing a media preparator, you will benefit from a 12-month warranty. Our commitment is to minimize any potential downtime in your laboratory in case of a breakdown. We have a wide range of spare parts in stock and, depending on your location, we may even provide you with a replacement equipment to ensure continuous operation.
10. Is it possible to perform IQ/OQ/PQ validation of the equipment?
RAYPA’s media preparators are industrial laboratory machinery that must be calibrated and inspected from time to time to ensure proper operation and performance. Also, if your laboratory operates under GLP/GMP environments, we inform you that equipment qualification can be performed, including the following:
- IQ which is the Installation Qualification
- OQ is the Operation Qualification
- PQ is the Performance Qualification
All three are intended to generate objective documentary evidence and provide proof of the suitability of installation, operation and performance of the equipment. We offer this service worldwide, either by ourselves or through our authorized partners.
We offer this service worldwide, either by ourselves or through our authorized partners. To perform them, in addition to reviewing the device documentation, we will perform tests on the microprocessor under normal operating conditions and also under operating limits to demonstrate its correct and constant performance. This documentation is available to any of our customers upon request.

11. Are there any training courses in order to get the most out of my equipment?
Yes, of course. After purchasing a media preparator, RAYPA offers a start-up service, as well as customized training sessions for customers that request it. These trainings can either be face-to-face or online. In addition, on our YouTube channel we offer you a video that explains the operation of our media preparators.
12. What is the equipment manufacturing and installation lead time? When can I start using it?
As a general rule, at RAYPA the manufacturing time for any of our media preparation models is usually 4 to 8 weeks. Although it can also be shorter if the reference is in stock.






